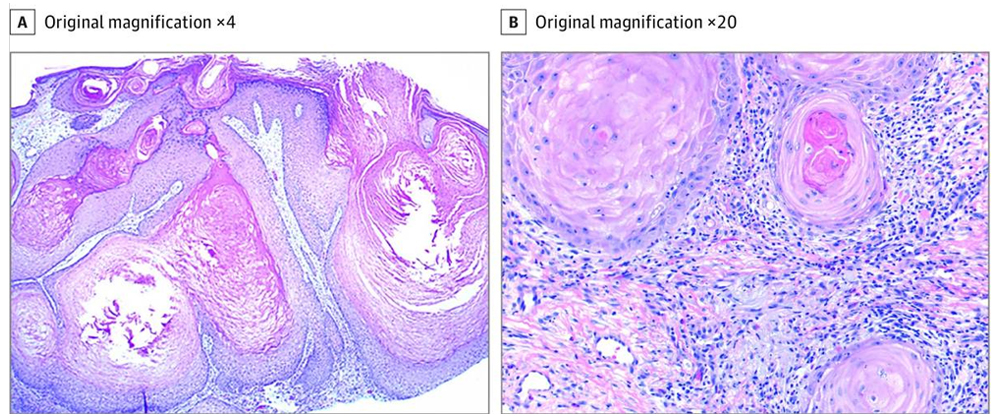
Pembrolizumab (Keytruda)
Pembrolizumab (Keytruda) has been shown to induce a variety of dermatologic adverse events including maculopapular eruption, lichenoid dermatitis, pruritus, vitiligo, and eruptive keratoacanthomas. An elderly patient with pulmonary adenocarcinoma treated with Pembrolizumab, who subsequently developed lichenoid eruptions and multiple cutaneous pseudoepitheliomatous hyperplasia. Up to eight skin biopsies are studied. The lesions are ranged from pseudoepitheliomatous hyperplasia with hyperkeratosis, hypergranulosis, diffuse to patchy lichenoid lymphocyte infiltrate with scattered eosinophils and neutrophils, confluent to scant dyskeratosis of the lower epidermis, minimal to overt invagination and cystic proliferation of squamous epithelium to papillomatosis with hypergranulosis and keratosis.
Overall the features of the lesions are lichenoid drug eruption-like, lichen planus-like, early invasive squamous cell carcinoma-like, early keratoacanthoma-like, and verruca-like. Multiple ulcers developed after radiation therapy. Five months later, the patient developed extensive lichenoid dermatitis presented as multiple pruritic red papules and nodules on her legs and arms bilaterally. In summary, Pembrolizumab could induce lichenoid eruption with pseudoepitheliomatous quality. It could be rather difficult to differentiate Pembrolizumab induced pseudoepitheliomatous reaction from an early invasive squamous cell carcinomas or keratoacanthomas clinically and pathologically.